Background :
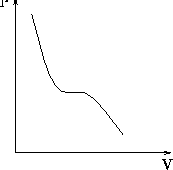
We are confronted daily with the phase transition from liquid to gas -- for example every time we boil water. Now we use the simulation to help our understanding of the microscopic properties of water that change when a phase transition occurs. At the boiling point of any liquid, a large fraction of molecules possess kinetic energy high enough to break bonds with the bulk liquid. That is, the pressure within the liquid is greater than the ``outside'' pressure and the liquid ``evaporates,'' resulting in a departure of molecules from the bulk fluid. The PV--phase diagram of water (Fig. 4.2.1) shows that as we lower the pressure, the volume increases. However this curve does not continue linearly; its decline comes to a halt and for a period the volume can increase without a change in pressure. The curve has a plateau in this region. Then the curve continues its downward slope. This interruption marks the phase transition from liquid to gas. When the liquid evaporates, its pressure does not rise, because the system expands at the same time (provided the the NPT ensemble has been chosen.)

Fig. 4.2.1 The PV--phase diagram of water.
System settings :
Start with the NPT ensemble. Fix the temperature at 300 K and the pressure
at 500 Mpa (Mega--pascal, 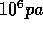 .) Raise the pressure in increments of 20 Mpa. When
you get close to the transition (on approaching the plateau in Fig. 4.2.1), decrease
the increment size. This ensures a lot of
data points in the transition region to correctly show the behaviour of the
system.
.) Raise the pressure in increments of 20 Mpa. When
you get close to the transition (on approaching the plateau in Fig. 4.2.1), decrease
the increment size. This ensures a lot of
data points in the transition region to correctly show the behaviour of the
system.
Questions :