

Next: Simulate the liquid--to--gas
Up: Simulations with the
Previous: Simulations with the
Background :
The unique physical and chemical behaviour of water is due to its
hydrogen bonds.
These bonds account for the extremely high boiling point of water in comparison
to other liquids.
The hydrogen sulfide molecule (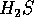 ), for example, is more massive than the
water molecule, has nearly the same geometry, and sulfide is in the
same group
), for example, is more massive than the
water molecule, has nearly the same geometry, and sulfide is in the
same group 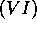 as oxygen in the table of elements.
However hydrogen sulfide is
a gas at room temperature and is not capable of making hydrogen bonds.
If water were a gas at room temperature, life
as we know it would be impossible.
The fact that water has its density maximum not in the solid
but in the liquid phase (at
as oxygen in the table of elements.
However hydrogen sulfide is
a gas at room temperature and is not capable of making hydrogen bonds.
If water were a gas at room temperature, life
as we know it would be impossible.
The fact that water has its density maximum not in the solid
but in the liquid phase (at 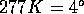 Celsius) results from
the fact that the network is
not static; rather it is a fluctuating network of flickering hydrogen bonds.
If this were not so -- if ice sank rather than floated, -- many of the
earth's oceans would be permanently frozen solid.
Celsius) results from
the fact that the network is
not static; rather it is a fluctuating network of flickering hydrogen bonds.
If this were not so -- if ice sank rather than floated, -- many of the
earth's oceans would be permanently frozen solid.
The hydrogen bond is not a normal bond;
it is a shared electron bond (see also section 3.1 on Bonds).
It can be regarded as a weak covalent bond, meaning that the energy needed to
break it is low - about 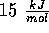 (compared with
(compared with 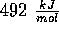 to break one H--O bond in H--O--H).
This is the reason why thermal agitation is sufficient to cause
hydrogen bonds to ``flicker'' on and off rapidly
in liquid water.
to break one H--O bond in H--O--H).
This is the reason why thermal agitation is sufficient to cause
hydrogen bonds to ``flicker'' on and off rapidly
in liquid water.
In this section we explore the influence that
heating and cooling have on the dynamics and
orientation of the hydrogen bond network.
System settings:
Start with the NVT ensemble. Set the temperature to 273 K and fix the
density at 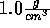 . Slowly raise the temperature in
increments of 10 K and view the changes that occur.
Pay attention to the ``flickering'' of the hydrogen bonds and notice the
increase in speed at which the water molecules rotate and move about.
. Slowly raise the temperature in
increments of 10 K and view the changes that occur.
Pay attention to the ``flickering'' of the hydrogen bonds and notice the
increase in speed at which the water molecules rotate and move about.
Questions :
- Explain why the H--bond is so special in comparison to other bonds.
- What is a fluctuating network? Compare this with a static network
(e.g. polymers).
- What influence does raising or lowering the temperature have
on the hydrogen bond network?
on the dynamics of the system?
on the orientation of the molecules?


Next: Simulate the liquid--to--gas
Up: Simulations with the
Previous: Simulations with the
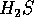 ), for example, is more massive than the
water molecule, has nearly the same geometry, and sulfide is in the
same group
), for example, is more massive than the
water molecule, has nearly the same geometry, and sulfide is in the
same group 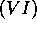 as oxygen in the table of elements.
However hydrogen sulfide is
a gas at room temperature and is not capable of making hydrogen bonds.
If water were a gas at room temperature, life
as we know it would be impossible.
The fact that water has its density maximum not in the solid
but in the liquid phase (at
as oxygen in the table of elements.
However hydrogen sulfide is
a gas at room temperature and is not capable of making hydrogen bonds.
If water were a gas at room temperature, life
as we know it would be impossible.
The fact that water has its density maximum not in the solid
but in the liquid phase (at 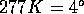 Celsius) results from
the fact that the network is
not static; rather it is a fluctuating network of flickering hydrogen bonds.
If this were not so -- if ice sank rather than floated, -- many of the
earth's oceans would be permanently frozen solid.
Celsius) results from
the fact that the network is
not static; rather it is a fluctuating network of flickering hydrogen bonds.
If this were not so -- if ice sank rather than floated, -- many of the
earth's oceans would be permanently frozen solid.
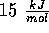 (compared with
(compared with 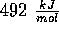 to break one H--O bond in H--O--H).
This is the reason why thermal agitation is sufficient to cause
hydrogen bonds to ``flicker'' on and off rapidly
in liquid water.
to break one H--O bond in H--O--H).
This is the reason why thermal agitation is sufficient to cause
hydrogen bonds to ``flicker'' on and off rapidly
in liquid water.
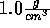 . Slowly raise the temperature in
increments of 10 K and view the changes that occur.
Pay attention to the ``flickering'' of the hydrogen bonds and notice the
increase in speed at which the water molecules rotate and move about.
. Slowly raise the temperature in
increments of 10 K and view the changes that occur.
Pay attention to the ``flickering'' of the hydrogen bonds and notice the
increase in speed at which the water molecules rotate and move about.