|
|
|
 |
|
|
|
|
| T | P | V | N | N/V | PV/NT | |
| A | ||||||
| B | ||||||
| C |
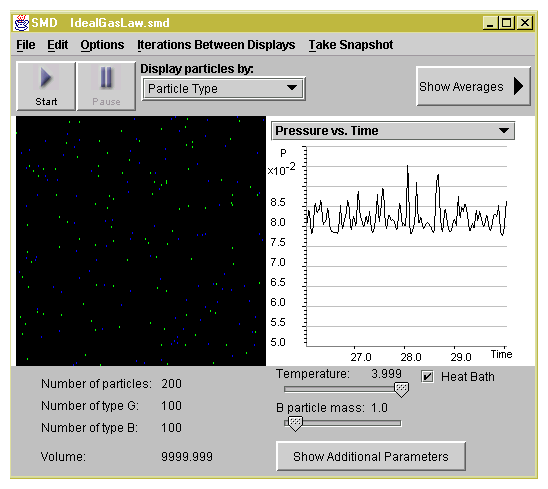
Set-up A: 100 green particles, 100 blue, mass = 1
Set-up B: 100 green particles, mass = 1
Set-up C: 100 blue particles, mass = 10
|
|
|
|
|
|
|
|
| T | P | V | N | N/V | PV/NT | |
| D | ||||||
| E | ||||||
| F |
Set-up D: 200 green, N/V=0.1
Set-up E: 200 green, N/V=0.2
Set-up F: 200 green, N/V=0.5
|
|
|
|
Now we will determine the
range of temperatures for which the ideal gas law equation is valid.
|
|
|
|
| T | P | V | N | N/V | PV/NT | |
| G | ||||||
| H | ||||||
| I |
Set-up G: 200 green, T=2
Set-up H: 200 green, T=1
Set-up I: 200 green, T=0.5
|
|
|