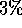 or less) then equilibrium has been reached.
or less) then equilibrium has been reached.
When investigating the microscopic properties of a system, we often refer to the fact that the system should be in equilibrium to ensure reproduceable and realistic results. Equilibrium means that the thermodynamic properties of the system, such as temperature and pressure, will not change when the system is left alone. Technically, equilibrium means that the system has found a potential energy minimum for a given set of thermodynamic properties. The system moves naturally toward a potential energy minimum:
When the Wasser program first starts,
it randomly distributes the molecules in the simulation box
on a cubic lattice and gives them mixed velocities and directions of motion.
As the program runs,
the molecules move about, interact, and
reorient themselves, automatically tending to a state of minimum potential energy.
Once this process no longer causes changes in the energy or thermodynamic properties
(such as temperature and pressure), we say that the system
is in equilibrium. The molecules still move about, but
in such a way that the potential energy stays near a minimum.
To check whether or not
equilibrium is established, we monitor the potential energy of a
system during a simulation. If it fluctuates by only a small amount
(about 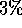 or less) then equilibrium has been reached.
or less) then equilibrium has been reached.