

Next: The hydration shell
Up: Simulations with the
Previous: Simulate the solid--to--liquid
So--called stretched water is water at a density lower that 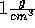 at normal room temperature. Experimentally one can stretch water by placing
it in a cylinder with a tight fitting piston and pulling the piston outward
without letting air into the system. This experiment is hard to do, because an
immense force is needed to expand the system significantly.
at normal room temperature. Experimentally one can stretch water by placing
it in a cylinder with a tight fitting piston and pulling the piston outward
without letting air into the system. This experiment is hard to do, because an
immense force is needed to expand the system significantly.
In contrast it is easy to simulate stretched water using the Wasser program.
Simply specify a density less than 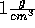 using the NVT ensemble or
set the pressure to a value smaller than 62 Mpa in the NPT ensemble.
using the NVT ensemble or
set the pressure to a value smaller than 62 Mpa in the NPT ensemble.
Background :
When looking at molecules of water at density 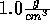 we see a randomly structured hydrogen bond network.
When we start decreasing the density,
the pressure of the system starts to decrease and gradually takes
negative values.
we see a randomly structured hydrogen bond network.
When we start decreasing the density,
the pressure of the system starts to decrease and gradually takes
negative values.
To understand negative pressure we can picture a rubber band that we hold
between our fingers. When we stretch the rubber band, it tries to snap
back to its relaxed position.
We can say that the ``outward force of the rubber band on our fingers
is negative.''
The same thing happens when we put water into a closed container without
bubbles then stretch the container. Both the container and the water try
to snap back to their relaxed conformation.
``Pressure'' ordinarily means ``outward force per unit area.''
In this sense, the pressure in the water goes negative.
Stretching the water makes a greater volume available to the molecules.
The stretched network strives for the energetically most favoured conformation.
This conformation is closely
related to that of ice, since a water molecule now, due to the tension,
has fewer nearest neighbors than it had at density 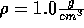 .
Under stretching, the system
will gradually go from a random network to the geometrically favoured open
hexagonal structure as the density is decreased.
If the density is lowered too far, however, the system reaches its
mechanical stability point; any further reduction in density
will ``tear the system apart.'' This occurs at a density of approximately
.
Under stretching, the system
will gradually go from a random network to the geometrically favoured open
hexagonal structure as the density is decreased.
If the density is lowered too far, however, the system reaches its
mechanical stability point; any further reduction in density
will ``tear the system apart.'' This occurs at a density of approximately
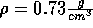 .
The system will no longer be in
the liquid phase, it will be like a droplet in vacuum.
.
The system will no longer be in
the liquid phase, it will be like a droplet in vacuum.
System settings :
Start with the NVT ensemble. Fix the temperature at 273 K and the density
at 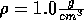 . Now slowly lower the density.
. Now slowly lower the density.
Questions :


Next: The hydration shell
Up: Simulations with the
Previous: Simulate the solid--to--liquid
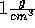 at normal room temperature. Experimentally one can stretch water by placing
it in a cylinder with a tight fitting piston and pulling the piston outward
without letting air into the system. This experiment is hard to do, because an
immense force is needed to expand the system significantly.
at normal room temperature. Experimentally one can stretch water by placing
it in a cylinder with a tight fitting piston and pulling the piston outward
without letting air into the system. This experiment is hard to do, because an
immense force is needed to expand the system significantly.
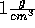 using the NVT ensemble or
set the pressure to a value smaller than 62 Mpa in the NPT ensemble.
using the NVT ensemble or
set the pressure to a value smaller than 62 Mpa in the NPT ensemble.
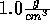 we see a randomly structured hydrogen bond network.
When we start decreasing the density,
the pressure of the system starts to decrease and gradually takes
negative values.
we see a randomly structured hydrogen bond network.
When we start decreasing the density,
the pressure of the system starts to decrease and gradually takes
negative values.
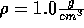 .
Under stretching, the system
will gradually go from a random network to the geometrically favoured open
hexagonal structure as the density is decreased.
If the density is lowered too far, however, the system reaches its
mechanical stability point; any further reduction in density
will ``tear the system apart.'' This occurs at a density of approximately
.
Under stretching, the system
will gradually go from a random network to the geometrically favoured open
hexagonal structure as the density is decreased.
If the density is lowered too far, however, the system reaches its
mechanical stability point; any further reduction in density
will ``tear the system apart.'' This occurs at a density of approximately
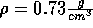 .
The system will no longer be in
the liquid phase, it will be like a droplet in vacuum.
.
The system will no longer be in
the liquid phase, it will be like a droplet in vacuum.
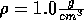 . Now slowly lower the density.
. Now slowly lower the density.